On March 12, 2024, Professor Zhu Bin's team from the School of Life Sciences at Huazhong University of Science and Technology (HUST) collaborated with Professor Wang Longfei's team from Wuhan University to publish a research paper titled "Structures and activation mechanism of the Gabija anti-phage system" in Nature.

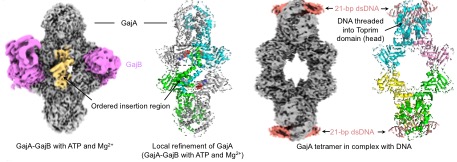
The Gabija immune system is the third most abundant prokaryotic immune system known in nature, following restriction-modification systems and CRISPR systems. Comprising only two genes, GajA and GajB, it provides efficient immunity against various virulent bacteriophages, making it one of the most broadly effective and concise immune systems in nature. The research group of Professor Zhu Bin, building on their long-term studies of nucleases, revealed the molecular mechanisms of the Gabija system for the first time. In 2021, they discovered that GajA is an NTP concentration-negatively regulated DNA nicking enzyme. Following phage invasion, the high levels of transcription reduce the NTP concentration within bacterial cells, activating GajA to cut both phage and bacterial DNA (Cheng et al., 2021, Nucleic Acids Research, doi: 10.1093/nar/gkab277). In 2023, they found that GajB is an A/GTP hydrolase activated by DNA termini, with its activation signal being a 3' end of a specific length. GajA and GajB mutually activate each other through a positive feedback loop characterized by synergistic responses to activation signals, leading to the degradation of cellular DNA and the depletion of important metabolites A/GTP, thereby causing abortive infection (Cheng et al., 2023, Cell Host & Microbe, doi: 10.1016/j.chom.2023.06.014). This study elucidated the cryo-electron microscopy structures of five states of the Gabija complex, detailing numerous molecular interactions involving ATP inhibition, DNA binding and cleavage, and GajA and GajB interactions. Recent high-impact papers, including two in Nature, have also reported on the cryo-EM structures of the Gabija complex, garnering significant interest in its immune mechanisms.
Zhu Bin and Wang Longfei are co-corresponding authors; doctoral student Li Jing from Wuhan University, postdoctoral researcher Cheng Rui from HUST, and associate researcher Wang Zhiming from Wuhan University are co-first authors of the paper. This research was supported by funding from the National Natural Science Foundation's Original Exploration Project and key research projects from Shenzhen.
Professor Zhu has focused on the fundamental research of microbial nucleic acid enzymes, guided by a philosophy of natural history, inspired by biodiversity. His team is committed to innovation and originality, striving to discover, collect, and analyze novel and unique microbial nucleases from environmental microorganisms. They aim to reveal new mechanisms of the central dogma and prokaryotic immune strategies based on enzyme functional characteristics while developing cutting-edge biotechnological tools. Notable discoveries include the GajA endonuclease regulated by nucleotide concentration, the GajB A/GTP hydrolase that senses DNA ends, and various novel enzymes from environmental microorganisms such as the VSW-3 RNA polymerase from high-altitude ice lakes, the Syn5 RNA polymerase from marine viruses, the NrS-1 DNA polymerase from the deep sea that does not require primers, and the only known m6A-dependent restriction enzyme of viral origin, etc. Zhu has published multiple research papers in top academic journals such as Nature, Nature Microbiology, Cell Host & Microbe, and PNAS. His research achievements have led to the commercialization of RNA synthesis tool enzymes like Ice lake™ RNA polymerase, Clean T7™ RNA polymerase, and T7 RNA polymerase 2.0 developed by companies such as Ribotech and Novoprotein.

